Mice not ‘furry little people’: Researchers rethink animal testing as human trials fail
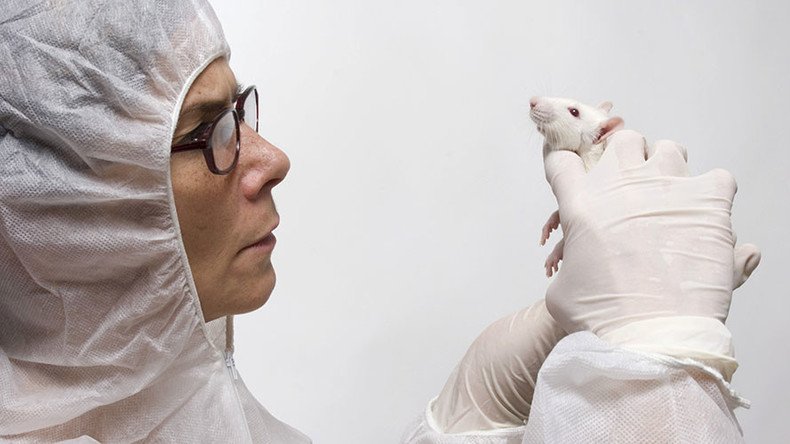
A group of biomedical researchers from Cornell, Stanford and Harvard universities are rethinking the tradition of relying on animal testing for new drugs, pointing out that many of those studies often fail in human trials, according to a new paper.
Relying on animal testing for new drugs has led to research that is not reproducible and translatable to human trials, the scientists said in a new paper on “Therioepistemology,” as they call the study of animal research.
“Maybe we need to stop thinking of animals as these little furry test tubes that can be or even should be controlled,” Joseph Garner, a behavioral scientist at the Stanford University Medical Center, and lead author of the study, told NPR. “And maybe instead we should think of them as patients.”
Quick turnaround for NPR. Medical waste in animal testing - cheers @mererizzo! #illustrationpic.twitter.com/EfWdp9Q8rL
— Sam Rowe (@_samdraws) April 10, 2017
Garner argues there is a worsening success rate in human trials ‒ currently 1 in 9 drugs entering human trials will succeed ‒ combined with an explosion of interest in reproducibility, which “has led to the growing suspicion that failure of translation from animal work to human outcomes may in some way reflect issues in animal research itself.”
Animal trials also raise ethical questions and often cost pharmaceutical companies thousands of dollars in research-and-development (R&D) losses when drugs fail.
The shift in thinking has developed over the past decade, as pharmaceutical companies noticed that every drug that fails in human trials worked on animals in the laboratory. That led to pharmaceutical companies disinvesting in internal animal R&D, and passing on the costs to startup and academic labs.
“Even this approach is not foolproof as pharmaceutical companies often cannot replicate the results of published work from academia,” Garner argued in the paper.
Software reduces need for animal testing https://t.co/yMkbnxmoRapic.twitter.com/5x8MeM78zr
— TEST Magazine (@testmagazine) March 31, 2017
The philosophy behind animal research is that everything has to be uniform as possible from one facility to another, Garner told NPR. However, studies are often conducted across regions – sometimes in different countries – and differences in cages, lighting conditions and ages of the mice lead to a huge amount of variation.
"Imagine you were doing a human drug trial and you said to the FDA [the Food and Drug Administration], ‘OK, I’m going to do this trial in 43-year-old white males in one small town in California’, where the men lived in identical ranch homes with the same monotonous diets and the same thermostat set to the same temperature," Garner told NPR.
“Which is too cold, and they can’t change it,” he added. “And oh, they all have the same grandfather!”
Newborn rats get mature hearts by serving as stem cell laboratory https://t.co/XeSkYBaRYGpic.twitter.com/yEaQirg4UT
— RT America (@RT_America) February 10, 2017
The FDA would laugh that off as an “insane setup,” Garner said, but it is what scientists try to control in trials and “as a result we learn absolutely nothing.”
Garner argues that mice trails might be more reliable if scientists recognized variety.
When scientists began using animals in research over a century ago, it was to study them as animals, not human stand-ins.
“As this process went on, people stopped seeing them as specialized animals and started seeing them more and more as prototypical mammals,” Todd Preuss, an anthropologist at the Yerkes National Primate Research Center at Emory University told NPR.
This was due in part to financial considerations, but also a belief that any disease could be cured by studying them.
That assumption overlooked the fact that rats and humans have been developing on their own evolutionary paths for tens of millions of years, with their own unique features, Preuss said.
The paper argues there is a value to the animal model as it offers a scientist a biomarker from birth to disease onset in a year or less (in the case of mice). That is less applicable when studying pain, where a mouse measurement of pain is largely based on reflexive or guarding responses but misses the emotional experience of pain itself felt by the human.
For neurological diseases, scientists might learn more from studying human cells than whole animals, said Gregory Petsko, who studies Alzheimer’s disease and other neurological disorders at the Weill Cornell Medical School.
Petsko argues animals are still useful for studying the safety of potential new treatments, but beyond that, he says, don’t count on them.
US Congress seeks to limit testing chemicals on live animals https://t.co/GMy2znXwtvpic.twitter.com/pOHcHPQUXS
— RT (@RT_com) June 9, 2016
Going a step further, a team at John Hopkins University announced in March they were conducting research to determine how useful testing on dogs, mice and other animals is in predicting whether drugs and chemicals are toxic to humans, according to the Baltimore Sun. The research could accelerate a push to end animal testing already underway for ethical and practical reasons.
One promising replacement for animal testing is “tissue on a chip” according to Kristie Sullivan of Physicians Committee for Responsible Medicine, a medical ethics group that opposes animal testing.
“We’re seeing more and more researchers trying to incorporate human-based methods into research, using human cells, stem cells or tissue on a chip,” Sullivan told the Washington Post. “The more of those methods used, the better for human health and animals.”
Advocates for animal testing argued that computer models and cell cultures reduce the number of animals used, but that there are limitations.
“[T]here is no way to completely replace animal research because the pathway to full replicating a complete living system does not yet exist,” said Matt Bailey, president of the Foundation for Biomedical Research, which advocates for animal testing.
Regulators at the FDA and the Environmental Protection Agency still argue animal testing is necessary.
Beating ‘heart-on-a-chip’ developed to replace human organs & animal testing (VIDEO) https://t.co/zGg6UYsuYtpic.twitter.com/LDVf79Pdrf
— RT (@RT_com) March 13, 2016
Many researchers hope to decrease the number of drugs that show promise in animal testing but fail to prove safe and effective in human trials, failures that are costly and disappointing to pharmaceutical companies and researchers as well as to patients hoping for better therapy and cures. A drug trial for a promising Alzheimer’s drug failed in a large trial last year.
More than 767,600 animals are used in research in 2015, according to date from the US Department of Agriculture. The number included dogs, cats, guinea pigs, hamsters, rabbits, primates and some farm animals – but not rats, mice or birds, which are the most common test subjects.
The paper, Introducing Therioepistemlogy: the study of how knowledge is gained from animal research was published this month in Nature America.