Azerbaijan approves trial of Covid-19 vaccine combination, set to test Russia’s Sputnik V together with AstraZeneca jab
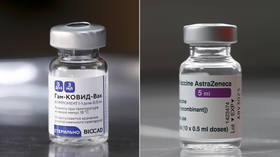
Azerbaijan has given the go-ahead to the world's first clinical trials combining two Covid-19 vaccines, with the country set to test inoculation using both Russia's Sputnik V and the AstraZeneca-Oxford University jab.
Cooperation between the two vaccine producers was first floated last December, when AstraZeneca accepted the proposal of the Russian Direct Investment Fund (RDIF) to begin testing how the two jabs combine. Now, Azerbaijan has stepped forward as being willing to be the first country to host the clinical trial, which will eventually take place over six months in several countries, with 100 volunteers recruited in each.
“We need to join our international efforts and use the most advanced solutions to defeat the coronavirus,” RDIF CEO Kirill Dmitriev said on Tuesday. “[Sputnik V] is one of only three vaccines in the world with efficacy of over 90 percent. We are ready to develop cooperation with other manufacturers to increase the number of affordable and effective vaccines.”
Also on rt.com Most Russians could be vaccinated against Covid-19 by summer, as Sputnik V creator reveals doses can be kept unfrozen for 2 monthsThe trial will be run jointly by the Gamaleya Center, the lab which made the Sputnik V vaccine, alongside AstraZeneca, RDIF, and Russian pharmaceutical company R-Pharm.
“R-Pharm has been actively working with the Republic of Azerbaijan for several years; we opened a modern production facility here in 2019, later in 2020 we registered two drugs from our anti-Covid portfolio: Artlegia and Coronavir,” said Alexey Repik, the chairman of R-Pharm's board of directors. “This pilot study is vital for developing a new approach to the prevention of Covid-19.”
The Sputnik V and the AstraZeneca vaccines use different vectors to form an immune response, and combining the two may generate broader protection through a more robust immune response and better accessibility.
Also on Tuesday, the RDIF revealed that the EU’s European Medicines Agency (EMA) accepted its application for registering Sputnik V in the bloc, with the scientific consultation process between Moscow and Brussels now completed.
In Russia itself, the vaccine is being used in the government's mass inoculation drive. Last week, Gamaleya Center head Alexander Gintsburg revealed that it will be possible to vaccinate the majority of Russia's population in the first six months of 2021.
“Given the level of the vaccine production that exists, I think that, by mid-year, we should achieve the necessary level of 60 percent of the [population] protected,” he said.
Like this story? Share it with a friend!